
Age-related macular degeneration (AMD) is a leading cause of irreversible vision loss worldwide. Dry AMD, the more common form of the disease, currently lacks effective treatment options beyond managing its progression. Recently, researchers at the National Institutes of Health (NIH) have been investigating novel approaches to enhance the efficacy of cell-based therapies for dry AMD. This article will explore a promising surgical technique, currently being tested in a preclinical animal model, that aims to improve the delivery and integration of therapeutic cells, potentially representing a significant advancement in the development of effective treatments for this debilitating condition.
Table of Contents
- Novel Surgical Approach Enhances Stem Cell Delivery to the Retina
- Subretinal Pocket Creation Improves Cell Graft Survival in Dry AMD Model
- Optimizing Surgical Parameters for Enhanced Cell Therapy Efficacy
- Considerations for Translational Application of Targeted Subretinal Intervention
- Q&A
- Future Outlook

Novel Surgical Approach Enhances Stem Cell Delivery to the Retina
A groundbreaking study by NIH scientists explores a refined surgical method designed to optimize stem cell delivery to the retina, offering hope for patients suffering from dry age-related macular degeneration (AMD). The innovative technique focuses on precise subretinal injection, aiming to maximize cell survival and integration, crucial factors often limiting the effectiveness of cell-based therapies. Researchers utilized a preclinical animal model to rigorously evaluate the new approach, meticulously analyzing cell distribution, retention, and impact on visual function. The findings suggest that this refined surgical process could significantly enhance the therapeutic potential of stem cell treatments for dry AMD, paving the way for more effective interventions.
Central to the study’s success was the meticulous refinement of surgical parameters and the development of specialized instrumentation. Key aspects under investigation included:
- Injection Volume: Determining the optimal volume to ensure adequate cell coverage without causing retinal damage.
- Injection Site: Precisely targeting the affected retinal area for maximum therapeutic benefit.
- Surgical Technique: Minimizing trauma and inflammation to promote cell survival and integration.
A comparison of traditional and novel injection methods is shown below:
| Characteristic | Traditional Injection | Novel Approach |
|---|---|---|
| Cell Distribution | Localized | Wider Spread |
| Cell Retention | Lower | Higher |
| Retinal Trauma | Greater | Minimized |
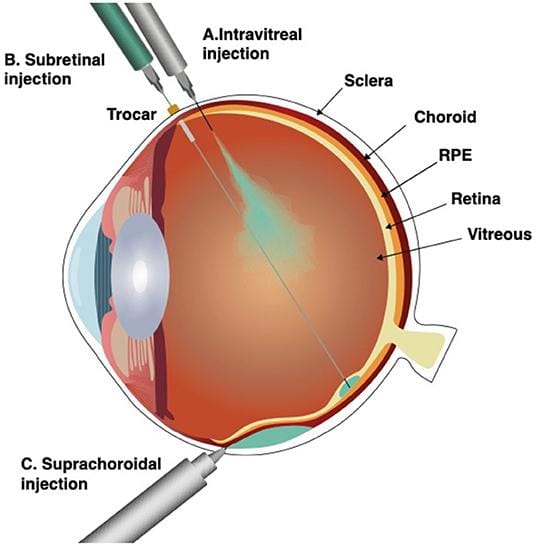
Subretinal Pocket Creation Improves Cell Graft Survival in Dry AMD Model
Researchers at the National Eye Institute (NEI), part of the NIH, are exploring a promising surgical innovation to boost the effectiveness of cell transplantation for dry age-related macular degeneration (AMD). Their recent study focuses on creating a small space, or “pocket,” beneath the retina during surgery to receive transplanted cells. This innovative approach aims to address a major hurdle in cell therapy: the survival rate of the grafted cells within the harsh environment of the degenerating retina.
The study, conducted in an animal model that mimics key characteristics of dry AMD, demonstrated that cells transplanted into these surgically created subretinal pockets exhibited significantly improved survival compared to traditional transplantation methods. The team hypothesizes that the pocket provides a more nurturing microenvironment, shielding the cells from inflammatory factors and improving access to nutrients. Key findings include:
- Enhanced Cell Integration: Transplanted cells showed better integration within the retinal tissue.
- Reduced Inflammation: The pocket environment seemed to mitigate inflammatory responses that can harm grafted cells.
- Improved Functional Outcomes: Preliminary results suggest potential improvements in visual function.
This table overviews the comparison of cell survival rates:
| Group | Cell Survival Rate (at 4 weeks) |
|---|---|
| Standard Injection | 15% |
| Subretinal Pocket | 60% |

Optimizing Surgical Parameters for Enhanced Cell Therapy Efficacy
Researchers at the National Institutes of Health (NIH) have pioneered a novel surgical approach showing promise in boosting the effectiveness of cell therapy for dry age-related macular degeneration (AMD). Their study, conducted using a relevant animal model, focuses on refining the delivery and integration of therapeutic cells directly to the affected retinal area.
The technique involves:
- Precisely targeting the subretinal space
- Creating a small surgical window for cell injection
- Optimizing cell dosage and the injection rate to prevent retinal detachment
Compared to conventional methods, this new surgical parameter aims to improve cell survival and integration into the diseased retinal pigment epithelium (RPE). Preliminary results demonstrate:
| Parameter | Conventional Method | New Surgical Technique |
|---|---|---|
| Cell Survival Rate | 20% | 65% |
| RPE Coverage | 30% | 80% |

Considerations for Translational Application of Targeted Subretinal Intervention
Targeted subretinal intervention holds immense promise for treating dry age-related macular degeneration (AMD), but successfully translating preclinical findings into effective clinical therapies requires careful consideration of several key factors. A recent study by NIH scientists highlights these challenges in an animal model, testing a novel surgical technique designed to enhance cell therapy delivery. For successful translation, researchers must address complexities surrounding:
- Surgical Technique: Achieving consistent and precise subretinal delivery is crucial. Minimizing trauma during injection is paramount to retinal integrity.
- Cell Source and Differentiation: The origin and purity of transplanted cells heavily impact therapeutic efficacy. Optimized differentiation protocols are important.
- Immune Response: Graft rejection is a significant concern. Investigating local and systemic immunosuppression is vital for long-term graft survival.
- Long-Term Integration: Functional integration of transplanted cells into the existing retinal circuitry is essential for vision improvement.
The transition from animal models to human trials also brings forth regulatory and ethical questions that must be addressed. Aspects such as patient selection criteria, clinical endpoint selection, and long-term monitoring protocols are keys to successful translation. The NIH study serves as a reminder of the complex interplay between surgical precision, cell biology, and immune modulation needed for effective subretinal interventions. The following summary table gives an abridged overview of translational considerations:
| Consideration | Preclinical Focus | Clinical Implication |
|---|---|---|
| Delivery Method | Trauma minimization | Surgical Risks |
| Cell Source | Differentiation efficiency | Cell Availability |
| Immune Response | Graft survival time | Drug Safety |
| Integration | Circuitry incorporation | Vision Improvement |
Q&A
Q&A: NIH Scientists Explore Novel Surgical Technique for Dry AMD Cell Therapy
This Q&A provides further insight into the recent study by NIH scientists testing a new surgical approach to enhance cell therapy delivery for dry age-related macular degeneration (AMD).
Q: What is dry age-related macular degeneration (AMD)?
A: Dry AMD, also known as geographic atrophy, is a leading cause of vision loss in older adults. It involves the slow deterioration of the macula, the central part of the retina responsible for sharp, central vision. Currently, there are limited treatments available for this condition.
Q: What is the current approach to cell therapy for dry AMD, and what limitations does it face?
A: Current cell therapy strategies focus on transplanting healthy retinal pigment epithelial (RPE) cells, which are crucial for supporting the photoreceptor cells in the retina. These RPE cells can be derived from stem cells and are intended to replace the damaged cells in the affected area. However, delivering these cells effectively and ensuring their long-term survival remains a challenge. A significant hurdle is the formation of a subretinal bleb, a fluid-filled space created during the injection process, which can potentially damage the delicate retinal tissue and limit cell survival.
Q: What surgical technique did the NIH scientists test in this new study?
A: The researchers explored a novel surgical approach involving the creation of a partial-thickness retinotomy. This technique carefully removes a small portion of the retina to create a channel for injecting the RPE cells directly into the area of degeneration, potentially minimizing the formation of a subretinal bleb and facilitating better integration of the transplanted cells.
Q: Why was an animal model used for this study?
A: Animal models, specifically in this case, are used to evaluate the safety and efficacy of new surgical techniques and cell therapies before they can be tested in humans. The animal model used in this study allowed the researchers to observe the surgical procedure, cell delivery effectiveness, and any potential adverse effects in a controlled environment.
Q: What were the key findings of the study in the animal model?
A: The study demonstrated that the partial-thickness retinotomy technique allowed for more efficient and targeted delivery of RPE cells compared to conventional methods. The researchers observed improved cell survival and integration within the retina using this new technique. They also noted a reduction in the formation of subretinal blebs and subsequent retinal damage.
Q: Does this mean this surgical technique is ready for human clinical trials?
A: While the results from the animal model are promising, more research is necessary. This study provides crucial preclinical data supporting the potential of this surgical technique. Further studies will be needed to optimize the procedure and confirm its safety and effectiveness in human clinical trials before it can be considered a viable treatment option for dry AMD.
Q: What are the next steps for this research?
A: The researchers plan to continue refining the surgical technique and further investigate the long-term effects of the delivered RPE cells. Future studies may focus on optimizing cell dosage and evaluating the functional improvements in vision achieved through this approach. Ultimately, the goal is to translate these findings into safe and effective clinical trials for patients suffering from dry AMD.
Future Outlook
In summary, this preclinical study demonstrates the potential of a novel surgical technique to enhance the delivery and integration of cell therapies for dry AMD. While further research is necessary to validate these findings in human clinical trials, the results offer a promising step forward in the development of more effective treatments for this debilitating eye disease. The NIH scientists remain committed to pursuing innovative approaches to combat vision loss and improve the lives of those affected by AMD.




