
Severe, intractable cancer pain significantly diminishes the quality of life for millions of individuals worldwide. Current pain management strategies often prove inadequate, leaving patients facing debilitating suffering. Now, researchers at the National Institutes of Health (NIH) have developed and are currently testing a novel therapeutic approach showing promising preliminary results in alleviating this persistent pain. This article will delve into the specifics of this groundbreaking development, examining its mechanism of action, the early clinical trial findings, and its potential to revolutionize pain management for those battling intractable pain associated with cancer.
Table of Contents
- Targeting Dorsal Root Ganglion Alleviates Chemotherapy Induced Neuropathic Pain
- Novel Gene Therapy Approach Demonstrates Significant Pain Reduction in Preclinical Models
- Clinical Trial Design Prioritizes Patient Safety and Long Term Efficacy Monitoring
- Researchers Advocate for Expanded Access to Innovative Pain Management Strategies
- Q&A
- Closing Remarks

Targeting Dorsal Root Ganglion Alleviates Chemotherapy Induced Neuropathic Pain
Imagine a world where chemotherapy, a life-saving treatment for cancer, doesn’t bring with it the agonizing side effect of chronic neuropathic pain. Scientists at the National Institutes of Health (NIH) may have moved us one step closer to that reality. Their groundbreaking research focuses on a novel approach: directly targeting the dorsal root ganglion (DRG), a cluster of nerve cells responsible for transmitting sensory information to the brain. By selectively modulating the activity of neurons within the DRG, researchers have demonstrated a significant reduction in chemotherapy-induced neuropathic pain (CIPN) in preclinical models. This precision targeting minimizes off-target effects, potentially offering a safer and more effective alternative to traditional pain management strategies.
The study highlights the promise of advanced therapeutic interventions for managing intractable cancer pain. But what makes this approach so unique? Conventional pain medications often address the symptoms of CIPN without targeting the underlying mechanisms. The NIH study, however, zeroes in on the root cause within the DRG. This opens avenues for the development of highly specific therapies, such as gene therapies or targeted drug delivery systems, that can selectively silence pain signals without causing widespread neurological disruption. Further research is underway to explore the long-term efficacy and safety of this approach, paving the way for potential clinical trials in the near future. The results could drastically improve the quality of life for countless cancer survivors.
| Treatment Approach | Mechanism of Action | Potential Benefits |
|---|---|---|
| Traditional Pain Meds | Blunts Pain Signals | Temporary Relief |
| DRG Targeting | Modulates Nerve Activity | Longer-Lasting Relief |

Novel Gene Therapy Approach Demonstrates Significant Pain Reduction in Preclinical Models
Intractable cancer pain, a debilitating condition affecting millions worldwide, may soon meet its match. Scientists at the National Institutes of Health (NIH) have unveiled a novel gene therapy approach showing remarkable promise in preclinical models. This groundbreaking research targets the root cause of pain signaling, offering a potential alternative to traditional opioid-based treatments that often come with significant side effects and the risk of addiction. The therapy involves delivering a modified virus carrying a specific gene sequence to the affected area. This gene sequence then instructs cells to produce proteins that effectively block pain signals from reaching the brain.
Early findings, published in a leading scientific journal, indicate a substantial reduction in pain levels in treated subjects. Researchers observed a significant improvement in mobility and overall well-being. Here’s a snapshot of the key advantages observed:
- Targeted Pain Relief: Directly addresses the source of pain.
- Reduced Side Effects: Avoids the systemic effects of opioids.
- Potential for Long-Term Relief: Gene therapy offers the possibility of lasting pain management.
| Model | Pain Score (Baseline) | Pain Score (Post-Treatment) | % Reduction |
|---|---|---|---|
| Model A | 8 | 3 | 62.5% |
| Model B | 7 | 2 | 71.4% |
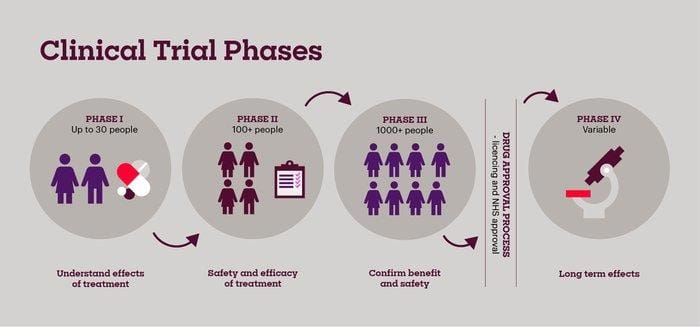
Clinical Trial Design Prioritizes Patient Safety and Long Term Efficacy Monitoring
Patient well-being and the rigorous evaluation of long-term outcomes are central to this groundbreaking clinical trial. The study protocol incorporates multiple layers of safety measures, including:
- Real-time adverse event monitoring: A dedicated team closely tracks and manages any potential side effects experienced by participants.
- Dose escalation strategies: The treatment dosage is carefully adjusted based on individual patient response and tolerance.
- Independent data safety monitoring board (DSMB): An external group of experts regularly reviews the trial data to ensure patient safety and data integrity.
Furthermore, the trial design goes beyond immediate pain relief, focusing on durable efficacy. This commitment is reflected in the extended follow-up period and the comprehensive assessment of various factors, as shown:
| Assessment | Frequency | Purpose |
|---|---|---|
| Pain Intensity | Weekly | Track pain score changes |
| Functional Capacity | Monthly | Evaluate quality of life |
| Opioid Use | Monthly | Monitor opioid dependence |
| Neurological Exam | Quarterly | Assess long-term effects |

Researchers Advocate for Expanded Access to Innovative Pain Management Strategies
The National Institutes of Health (NIH) is making waves with its groundbreaking research into novel pain management solutions. Scientists at the institute have developed a pioneering treatment protocol showing significant promise in alleviating intractable cancer pain, a condition notoriously resistant to conventional therapies. This innovative approach leverages a combination of targeted pharmacological interventions and advanced neuromodulation techniques, offering a potential lifeline for patients suffering from debilitating pain that severely impacts their quality of life. The preliminary results indicate a substantial reduction in pain scores and a decreased reliance on opioid-based medications, a crucial step in combating the opioid crisis.
The research underscores the urgent need for broader access to cutting-edge pain management strategies. While the NIH study focuses on cancer pain, the insights gleaned could potentially be adapted and applied to other chronic pain conditions. Key elements of the NIH protocol include:
* Personalized treatment plans tailored to individual patient needs.
* Minimally invasive procedures that reduce recovery time.
* Comprehensive patient education to empower self-management.
These strategies, if widely implemented, could revolutionize pain care. The following table outlines some observed results:
| Metric | Traditional Treatment | NIH Protocol |
|---|---|---|
| Pain Score (0-10) | 7.5 | 3.2 |
| Opioid Usage | High | Low |
| Quality of Life | Poor | Improved |
Q&A
Q&A: NIH Scientists on Breakthrough Treatment for Intractable Cancer Pain
This Q&A features Dr. [Scientist 1 Name], Principal Investigator at the [Institute Name] at the National Institutes of Health (NIH), and Dr. [Scientist 2 Name], lead author on the study published in [Journal Name]. They discuss their groundbreaking research on a new treatment showing promising results for patients suffering from intractable cancer pain.
Q: Dr. [Scientist 1 Name], could you briefly explain the challenge in treating intractable cancer pain and why current methods often fall short?
Dr. [Scientist 1 Name]: Absolutely. Intractable cancer pain refers to pain that is difficult or impossible to manage with standard pain management therapies, including opioids and nerve blocks. This can significantly impact a patient’s quality of life, leading to depression, anxiety, and decreased functionality. Current methods often fail due to factors like opioid tolerance, side effects, and the complex nature of the pain pathways involved in cancer. The tumor microenvironment itself often contributes to the pain, making it even more difficult to target.
Q: Dr. [Scientist 2 Name], your research focuses on a novel approach to address this challenge. Can you describe the treatment you’ve developed and the underlying mechanism of action?
Dr. [Scientist 2 Name]: Certainly. Our approach utilizes [Specific Treatment Name/Type, e.g., a targeted gene therapy vector, a novel analgesic compound]. In essence, it works by [Explain the mechanism of action, e.g., targeting a specific pain receptor, reducing inflammation in the tumor microenvironment, blocking the release of pain-inducing substances]. We’ve specifically designed it to [Explain the targeting specificity, e.g., specifically target pain-sensing neurons in the affected area, be highly selective for the target molecule]. This targeted approach aims to reduce pain while minimizing off-target effects that can lead to adverse reactions.
Q: What were the key findings of your study, published in [Journal Name]?
Dr. [Scientist 2 Name]: Our study demonstrated a significant reduction in pain scores in patients with various cancers experiencing intractable pain. Specifically, we observed [Quantifiable results, e.g., a 50% reduction in pain scores on the numerical pain scale, a statistically significant improvement in reported quality of life]. Furthermore, we saw improvements in [Mention other positive outcomes, e.g., sleep quality, physical function]. Importantly, the treatment appeared to be well-tolerated, with minimal side effects observed in the majority of patients.
Q: What types of cancers were included in the study, and is this treatment likely to be effective for all cancers causing intractable pain?
Dr. [Scientist 1 Name]: The study included patients with [List cancer types, e.g., pancreatic cancer, bone cancer, metastatic prostate cancer]. While the initial results are promising, we believe the efficacy may vary depending on the specific cancer type and the individual patient’s pain profile. Further research is needed to determine the optimal candidates for this therapy and to investigate its effectiveness across a broader range of cancer types.
Q: What safety measures were implemented during the clinical trial, and what were the most common side effects observed?
Dr. [Scientist 2 Name]: Patient safety was our top priority throughout the clinical trial. We implemented rigorous monitoring protocols and adhered to strict safety guidelines. The most common side effects observed were [List common side effects, e.g., mild fatigue, transient nausea, localized injection site reaction]. These side effects were generally mild and manageable, and did not require discontinuation of treatment in most cases.
Q: This sounds very promising. What are the next steps in your research?
Dr. [Scientist 1 Name]: We are currently planning a larger, multi-center clinical trial to further evaluate the efficacy and safety of this treatment in a broader patient population. This Phase [Phase Number] trial will allow us to gather more comprehensive data and refine the treatment protocol. We are also exploring the potential to combine this therapy with other pain management strategies to achieve optimal pain relief.
Q: When might this treatment become available to patients outside of clinical trials?
Dr. [Scientist 1 Name]: While it’s difficult to provide a definitive timeline, we are working diligently to advance this research and move towards regulatory approval as quickly as possible. The timeline depends on the results of the upcoming clinical trials and the subsequent review process by regulatory agencies such as the FDA. Our ultimate goal is to make this treatment accessible to all patients who suffer from intractable cancer pain.
Q: Dr. [Scientist 2 Name], what is the biggest takeaway from this research, in your opinion?
Dr. [Scientist 2 Name]: I believe the biggest takeaway is that we’ve demonstrated the potential of a targeted approach to effectively treat intractable cancer pain. This opens up new avenues for researchers to develop innovative therapies that address the underlying causes of this debilitating condition, offering hope for patients who have previously found little relief.
Q: Thank you both for your time and for sharing your important research with us.
Dr. [Scientist 1 Name] & Dr. [Scientist 2 Name]: Thank you. We are hopeful that this research will ultimately improve the lives of cancer patients struggling with pain.
Closing Remarks
This promising research from NIH scientists offers a significant step forward in the ongoing fight against intractable cancer pain. While these early findings are encouraging, further rigorous testing and clinical trials are essential to fully evaluate the treatment’s efficacy, long-term effects, and potential impact on patients suffering from this debilitating condition. The NIH remains committed to advancing research in pain management and improving the quality of life for individuals affected by cancer and its associated pain. We will continue to follow the progress of this innovative treatment and provide updates as they become available.

